SO4 2 Molecular Geometry / Shape and Bond Angles YouTube

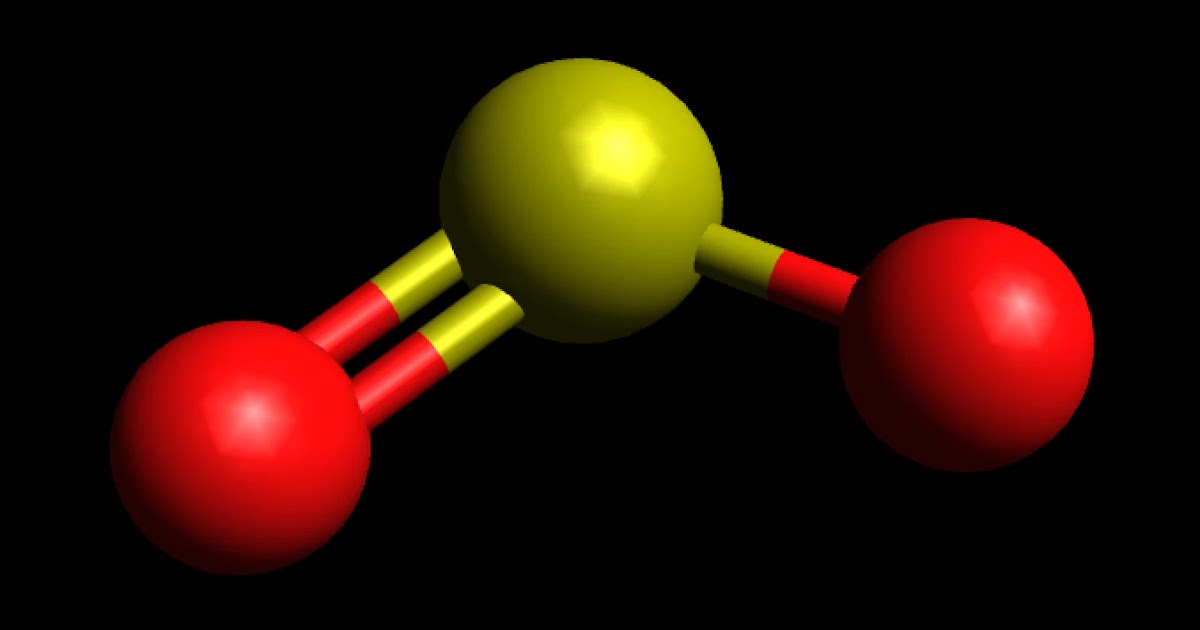
Is SO2 Polar or Nonpolar? Techiescientist
Page Contents show How to draw lewis structure of SO42-? The Lewis structure of a sulfate [SO4]2- ion consists of 1 sulfur (S) atom and 4 atoms of oxygen (O). The sulfur atom is present at the center of the Lewis structure while the oxygen atoms occupy terminal positions.
MakeTheBrainHappy Is SO2 Polar or Nonpolar?
Figure \(\PageIndex{6}\): The molecular geometry of a molecule affects its polarity. CO 2 is a nonpolar molecule, while H 2 O is a polar molecule. It should be noted that if the all of the bonds in a molecule are nonpolar, then the molecule will be nonpolar. Consider a molecule of O 2, . The double bond between the two oxygen atoms is a.

SO42 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
Each C-O bond in CO 2 is polar, yet experiments show that the CO 2 molecule has no dipole moment. Because the two C-O bond dipoles in CO 2 are equal in magnitude and oriented at 180° to each other, they cancel. As a result, the CO 2 molecule has no net dipole moment even though it has a substantial separation of charge.

Lewis Structure SO4 2 plus dipoles, shape, angles, resonance and
Henry Agnew (UC Davis) 5.10: Electronegativity and Bond Polarity is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Covalent bonds can be nonpolar or polar, depending on the electronegativities of the atoms involved. Covalent bonds can be broken if energy is added to a molecule.

Is SO2 Polar or Nonpolar? Molecular polarity for SO2 Dr K YouTube
Sulfate ion (SO42-) is one of the most common ions that people in chemistry need to deal with. This is a polyatomic anion having a negative charge of -2. We find sulfates in a wide range of compounds, some of the well-known being MgSO4, CaSO4, Na2SO4, and PbSO4. We can easily prepare sulfates via oxidizing metal sulfites and sulfides.

So42 Lewis Structure Molecular Geometry slidesharetrick
Learn to determine if SO42- is polar or nonpolar based on the polarity between bonds and the molecular geometry (shape).Ions, like SO42- (sulfate) are someti.
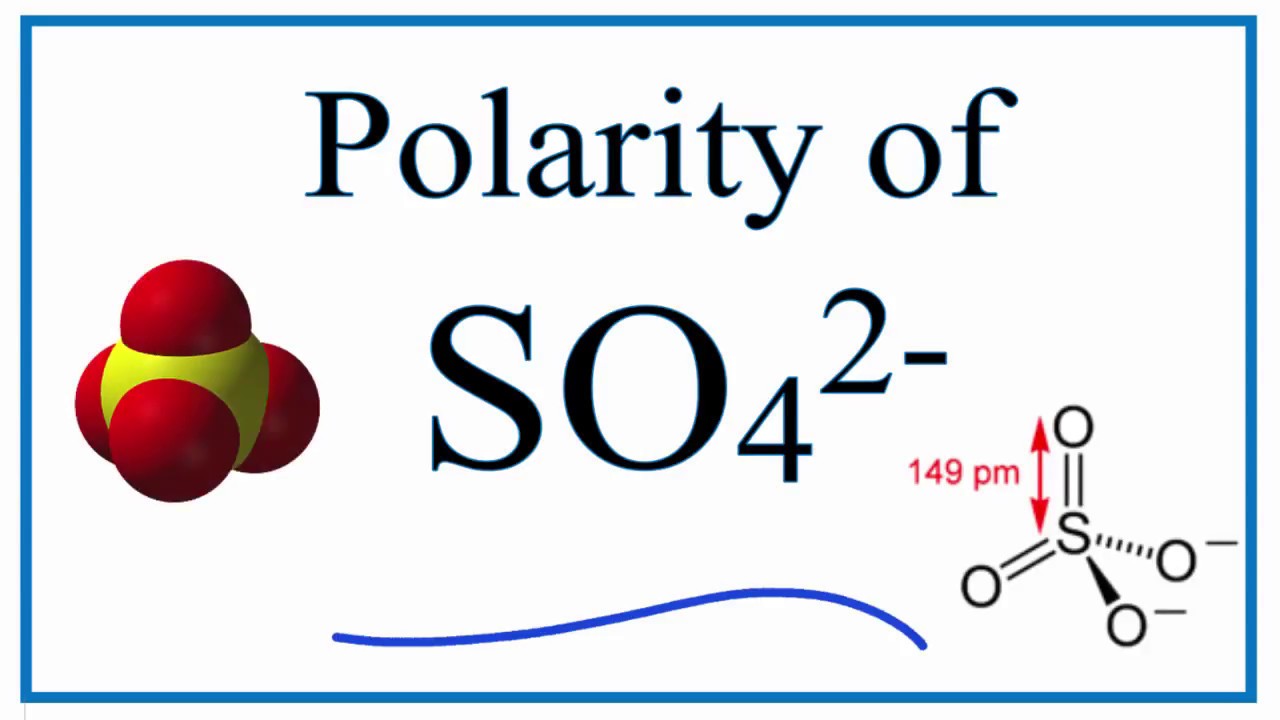
Is SO42 Polar or Nonpolar? (sulfate ion) YouTube
Learn to determine if SO2 (Sulfur dioxide) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Stru.

Is SF4 Polar or Nonpolar? (Sulfur Tetrafluoride) YouTube
Is SO42- Polar or Nonpolar? (Sulfate Ion) Geometry of Molecules 2.71K subscribers 5 679 views 1 year ago Polarity of Molecules Hello Guys! SO42- ion or Sulphate ion's polarity is quite.
Chf3 Polar Or Non Polar
Notice that a tetrahedral molecule such as CCl4 CCl 4 is nonpolar Figure ( 4.14.1 4.14. 1. Another non polar molecule shown below is boron trifluoride, BF 3. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Figure 4.14.1 4.14. 1 Some examples of nonpolar molecules based on molecular geometry (BF 3 and CCl 4 ).
Solved Part II Short Answer. Complete according to
The SOF4 lewis structure consists of sulphur, oxygen and fluorine atoms having 6, 6 and 7 electrons respectively. Therefore, total valence electrons in SOF4 molecule is 6 (S) + 6 (O) + 7 x 4 (F) = 40. Hence SOF4 molecule has total forty valence electrons present on it. Also if we calculate total electron pairs of SOF4 then 40 / 2 = 20, we have.

Lewis Dot Diagram For So4 2
SO4^2-: - The 3D sketch for SO4^2- is a tetrahedral shape, with bond angles of approximately 109.5 degrees. AsF5: - The 3D sketch for AsF5 is a trigonal bipyramidal shape, with bond angles of approximately 90 and 120 degrees. Answer Step 3: Finally, we need to determine if each molecule is polar or nonpolar.
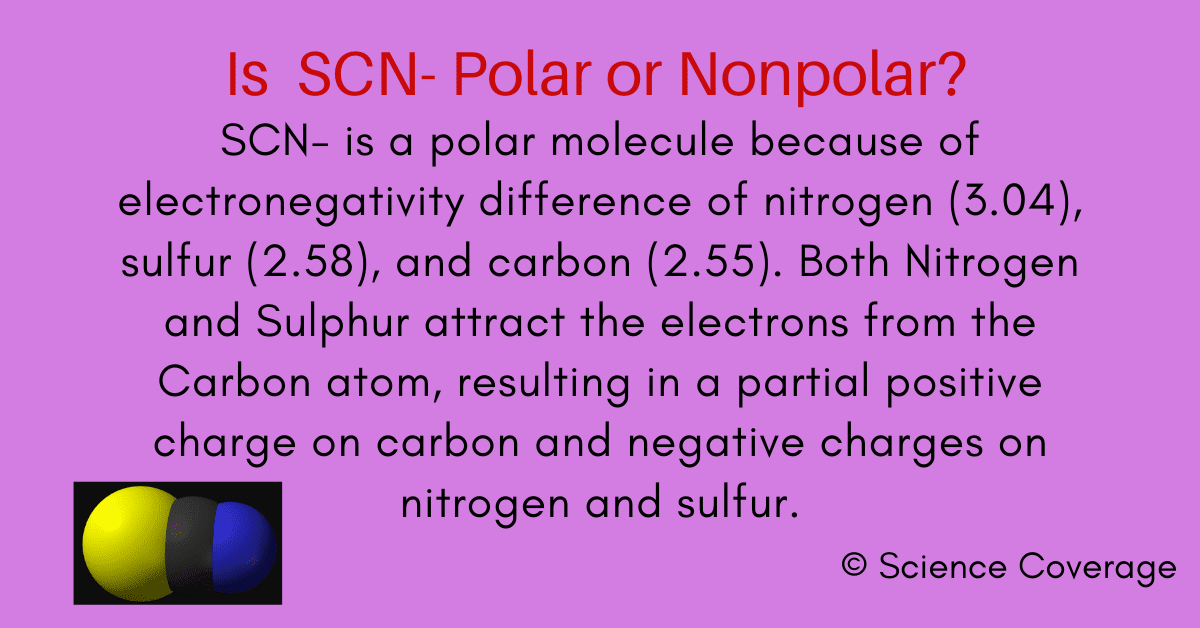
Is SCN Polar or Nonpolar?
Sulfate is a very weak oxidizing agent. Since sulfur is in its maximum oxidation number in sulfate ion, this ion cannot act as a reducing agent. This page titled Sulfate Ion (SO₄²⁻) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by James P. Birk. Sulfate ion is a very weak base.

Hướng dẫn vẽ cấu trúc Lewis của so4 2 lewis structure chi tiết và dễ hiểu
Answer = SO4 2- ( sulfate ) is Nonpolar What is polar and non-polar? Polar "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms.

Is SO2 Polar or Nonpolar? Techiescientist
SO42- is a chemical formula for Sulfate ion; it comprises one Sulfur Atom and four oxygen atoms. It also has a -2 charge because of the additional electrons it accepts to attain this structure. This blog post will help you understand if this ion is polar or nonpolar, although a -2 charge might confuse you.
SOLVED What is the Molecular Geometry and Polarity of SO42? a Linear
Draw the most important Lewis structure for [ SO4 ]2− and then answer the following questions. The underlined atom is the central atom. All other atoms are bonded directly to the central atom. (a) What is the electron-group geometry, according to VSEPR theory? (b) What is the molecular geometry? (c) Is this species polar or nonpolar?

Best Overview Is SO2 Polar or Nonpolar Science Education and Tutorials
SO42- is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of -2. It is a polyatomic anion and is used widely to synthesize other sulfates such as Zinc Sulfates, Magnesium sulfates, Iron sulfates, and much more. It is also a sulfate salt for sulphuric acids.