Free download hd /best overview is ch4 polar or nonpolar science

Discover 66+ draw the structure of clf3 super hot xkldase.edu.vn
ClF3 is a POLAR molecule because the Fluorine (F) present in the molecule is more electronegative, which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. These ẟ+ and ẟ- charges are responsible to make the entire ClF3 molecule polar.
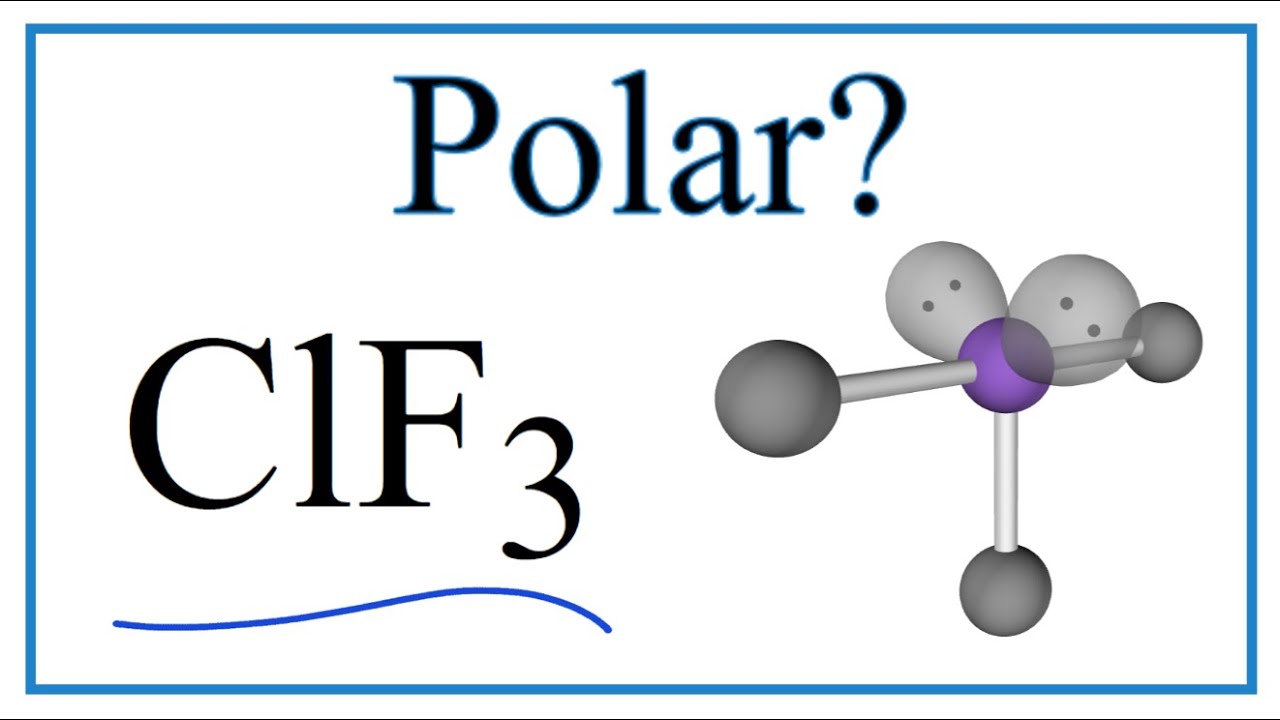
Is ClF3 Polar or Nonpolar (Chlorine trifluoride) YouTube
Figure 6.2.1 (a) The distribution of electron density in the HCl molecule is uneven. The electron density is greater around the chlorine nucleus. The small, black dots in the center of the green spheres indicate the location of the hydrogen and chlorine nuclei in the molecule. (b) Symbols δ+ δ + and δ− δ − indicate the polarity of the H.

Best Explanation CH2Cl2 polar or nonpolar [N01] Science Education
Hello Everyone! Welcome back to our channel, and for today's video, we will help you determine if Chlorine Fluoride is a polar or nonpolar molecule. To assess its polarity, we first look at the.

ClF3 is a polar and planar molecule Chemistry Chemical Bonding and
Chlorine trifluoride or ClF3 is an extremely reactive chemical compound with several varied applications and unique physical and chemical compounds. An interhalogen compound having both Cl and F, it has a density of around 3.79 g/l and a molar mass of 92.45 g/mol.
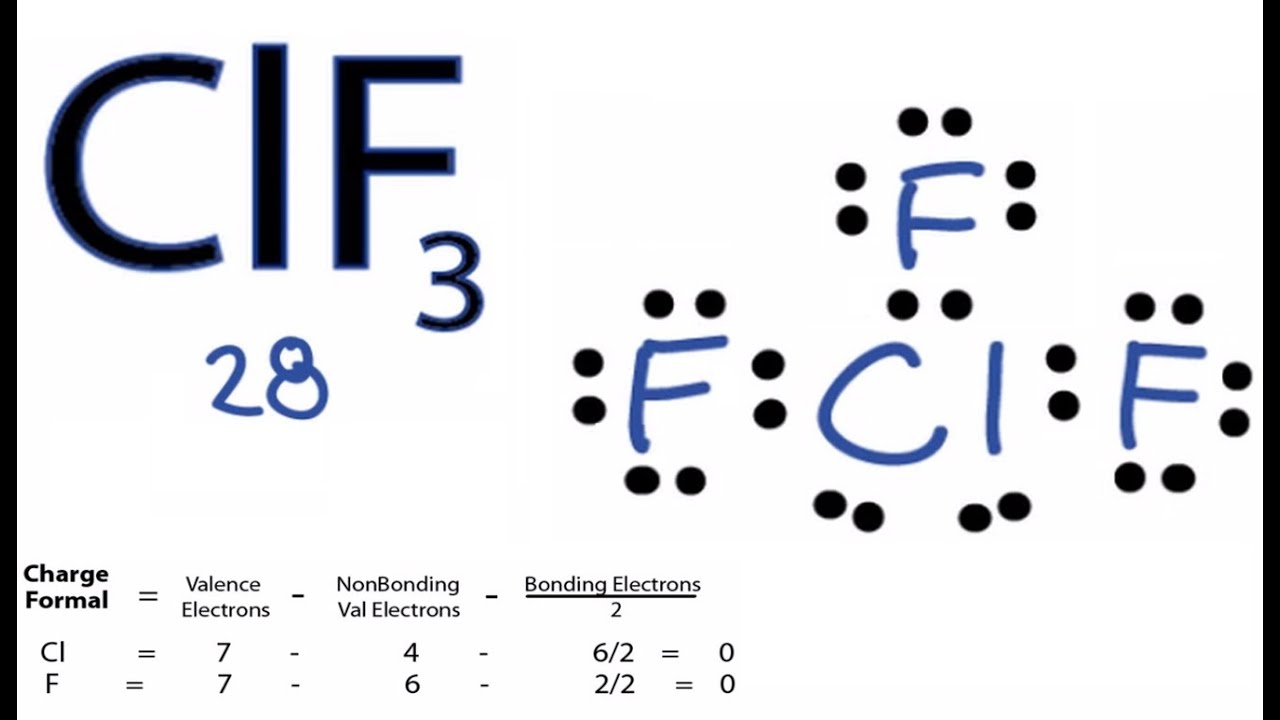
Clf3 Polar Or Nonpolar Asking List
Since there are three fluorine atoms in ClF3, calculate the total valence electrons as follows: 7 (chlorine) + 3 * 7 (fluorine) = 28 valence electrons. 2. Select the Central Atom In ClF3, the central atom is typically the least electronegative atom, which, in this case, is chlorine (Cl). 3. Connect Atoms by Placing Electron Pairs Between Them

Free download hd /best overview is ch4 polar or nonpolar science
ClF3 is a polar compound. sicl4 polar or nonpolar Is Nh3 Polar? Frequently Asked Questions

Is ClF3 Polar or Nonpolar (Chlorine Trifluoride) YouTube
ClF is a POLAR molecule because any two bonding atoms whose electronegativity difference is between 0.4 to 2.0 forms a polar bond. Here in ClF molecule, the electronegativity difference of Chlorine atom (Cl = 3.16) and Fluorine atom (F = 3.98) is 0.82 (i.e 3.98 - 3.16 = 0.82).

BrF3 Polar or Nonpolar? (Bromine Trifluoride) Molecules, Chemical
Learn to determine if ClF3 (Chlorine trifluoride) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lew.

ClF3 Molecular Geometry, Bond Angles & Electron Geometry (Chlorine
ClF3 is polar. The physical shape of the molecule is almost T-shaped and therefore given the two lone pairs of electrons also present on the molecule, the charge does not centre itself on the.
SOLVED Which molecule has polar bonds but is nonpolar? A. ClF3 B. H20
ClF3 or Chlorine Trifluoride is a strong fluorinating agent. To find out whether this molecule is polar or nonpolar, watch this video, where we share our quick and detailed method to.
Is ClF3 Polar or Nonpolar ? دیدئو dideo
How to draw lewis structure for ClF3? ClF3 lewis structure comprises three fluorine (F) atoms and one chlorine (Cl) atom. The chlorine (Cl) atom is kept at the central position and the fluorine (F) atoms are in the surrounding position in the lewis diagram. The lewis dot structure of ClF3 contains total of 11 lone pairs and 3 bond pairs.

How to draw ClF3 Lewis Structure? 4
When you place a molecule with an electric dipole in an electric field, a force acts to turn the molecule so that the positive and negative ends line up with the field. The magnitude of the turning force is given by the formula. µ = q × d. where q is the amount of charge and d is the distance between the two charges. µ is the turning moment.

Lewis Structures, VSEPR and Polarity Notes YouTube
Chlorine trifluoride is an interhalogen compound with the formula ClF 3.This colorless, poisonous, corrosive, and extremely reactive gas condenses to a pale-greenish yellow liquid, the form in which it is most often sold (pressurized at room temperature). Despite being famous for its extreme oxidation properties and igniting many things, chlorine trifluoride is not combustible itself.
MakeTheBrainHappy Is ClF3 Polar or Nonpolar?
Answer: ClF3 is a polar molecule due to the presence of two pairs of lone pair electrons. The resulting electron-electron repulsion causes a bent structure, leading to an unequal distribution of charge. This induces a permanent dipole.

Brf3 Polar Or Nonpolar
Why Does ClF3 Have a Large Dipole? The large dipole moment of ClF3 can be attributed to the molecule's polar bonds and its T-shaped molecular geometry. A dipole moment is a measure of the separation of positive and negative charges within a molecule. In ClF3, the chlorine atom is more electronegative than the fluorine atoms, resulting in.
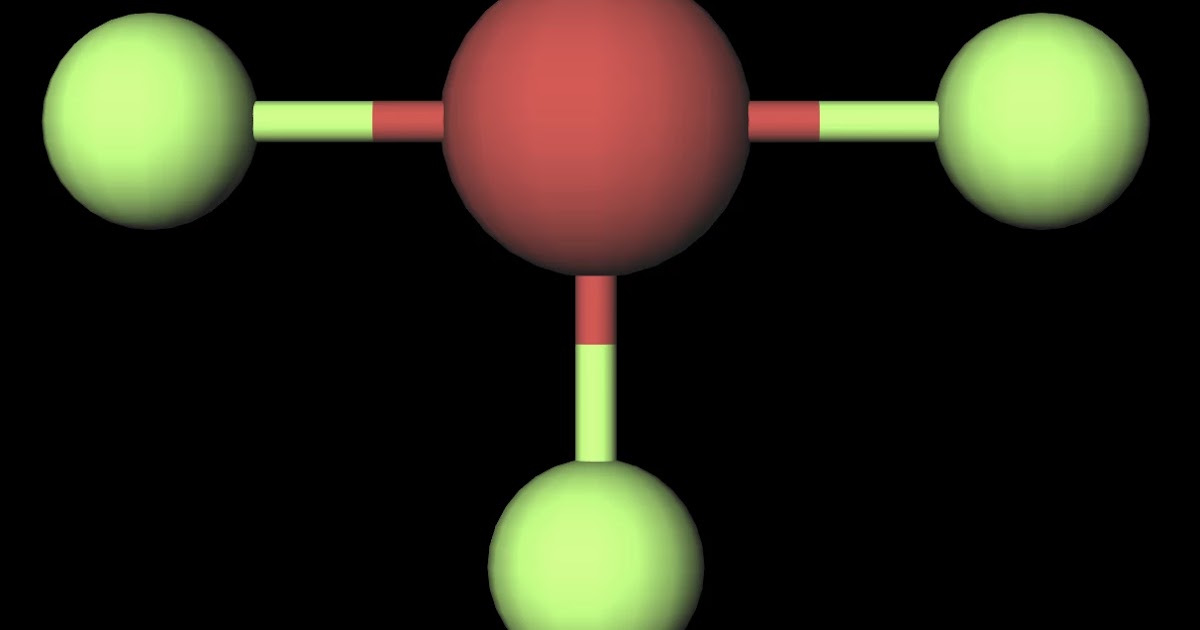
MakeTheBrainHappy Is BrF3 Polar or Nonpolar?
Expert-verified. Step 1. ANSWER. ClF A 3 molecule is Polar. View the full answer Step 2. Unlock. Step 3. Unlock. Answer.