konuşkan Sıçrama karar bohr modeli

Bohr's atomic model 100 years old South China Morning Post
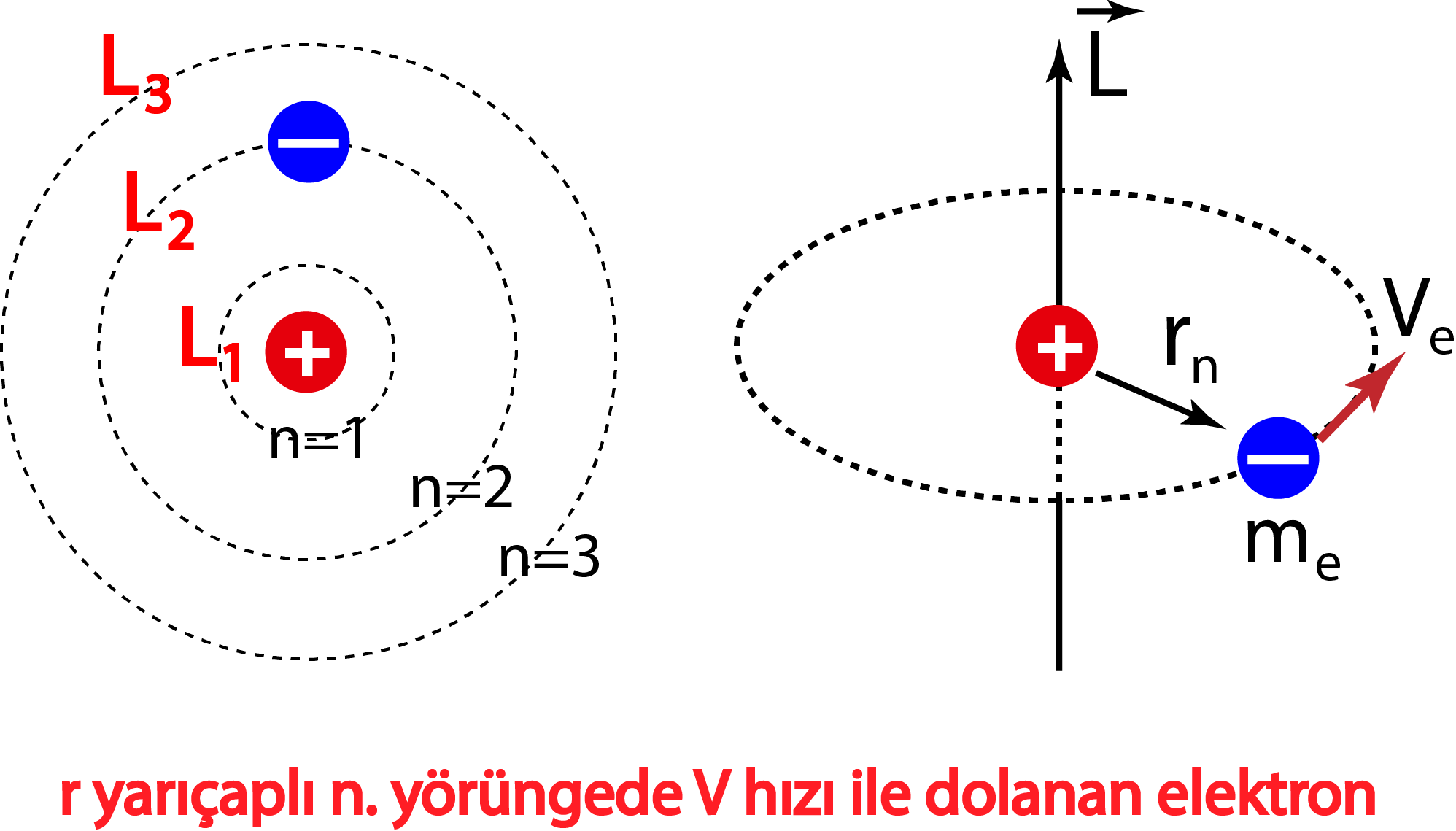
In 1913, Danish physicist Niels Bohr applied Max Planck's quantum theory to the nuclear atom of Ernest Rutherford, thus formulating the well-known planetary model of the atom, wherein electrons orbit a central nucleus in well-defined levels of energy ().Note that Bohr stated that electrons in the atom follow elliptical orbits (not circles as is often pictured).
ATOM MODELİ YAPILIŞI.Basit Atom Modeli yapımı.Atom modeli nasıl yapılır?Rutherford,Bohr. YouTube
Examples on Bohr Atomic Model. Example 1: Calculate the maximum number of electrons an o shell can hold. Solution: We know that O shell means 5th shell. Therefore, n=5. Applying the formula 2n 2 = 2 x 5 2 = 50. Thus, the maximum number of electrons O shell can hold is 50.

Atom Modeli YouTube
Figure 6.2.1 6.2. 1: Quantum numbers and energy levels in a hydrogen atom. The more negative the calculated value, the lower the energy. We can relate the energy of electrons in atoms to what we learned previously about energy. The law of conservation of energy says that we can neither create nor destroy energy.

Atom Modeli 3D (Atom Model) YouTube
ATOM MODELİ YAPILIŞI.Basit Atom Modeli yapımı.Atom modeli nasıl yapılır?Rutherford,Bohr.

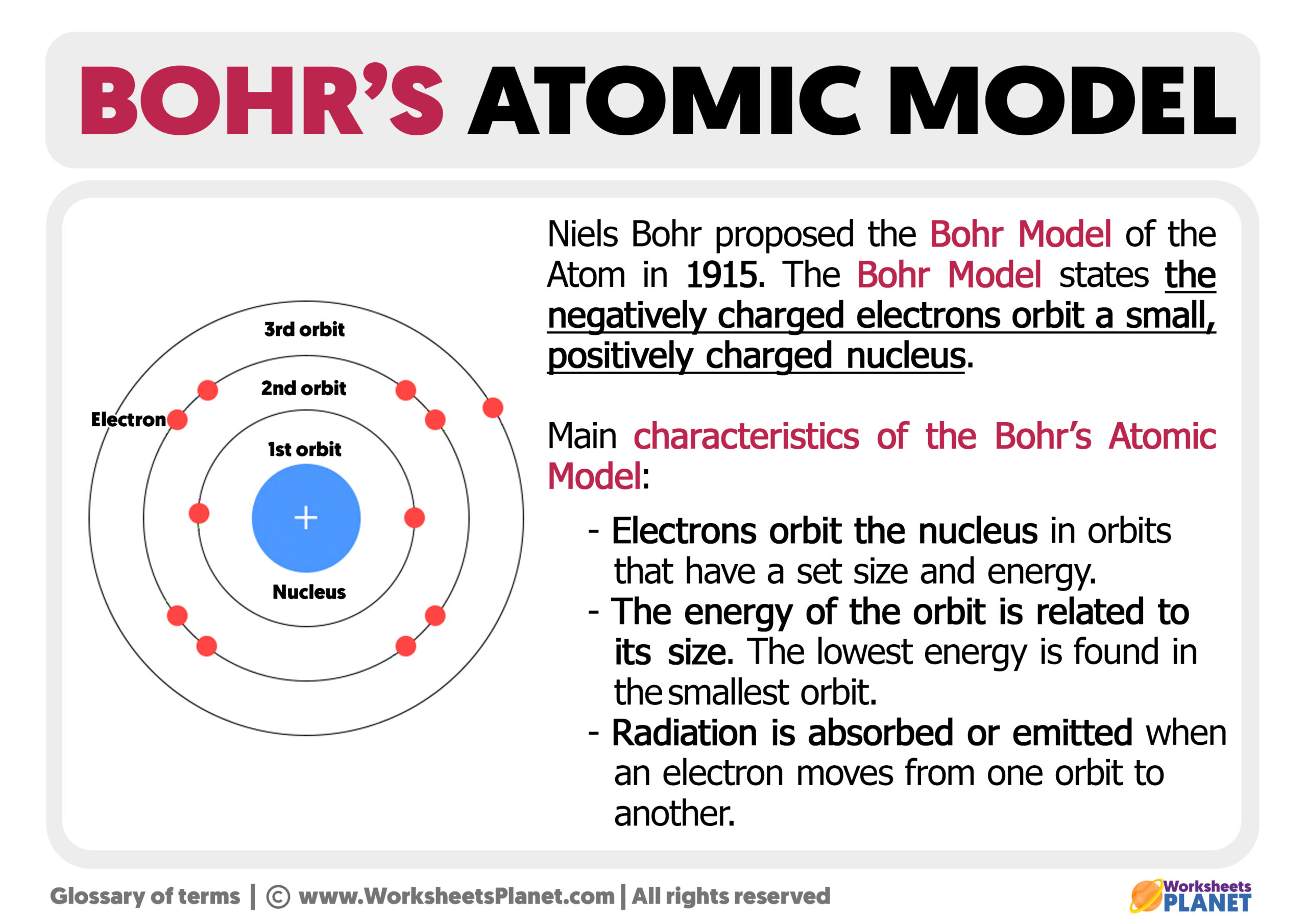
Bohr's Atomic Model Postulates Diagram Limitations
The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell model. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory.
:max_bytes(150000):strip_icc()/atomic-structure-conceptual-artwork-99312661-58af58c75f9b5860467ff472.jpg)
History of Atomic Theory
Bohr's model calculated the following energies for an electron in the shell, n. . : E ( n) = − 1 n 2 ⋅ 13.6 eV. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the photon energy is. h ν = Δ E = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 eV.

Strafor köpük çöp şiş bakır tel ve alüminyum folyo kullanarak Bohr lityum Atom Modeli oluşturduk
Atoms and Electrons Electronics Reference
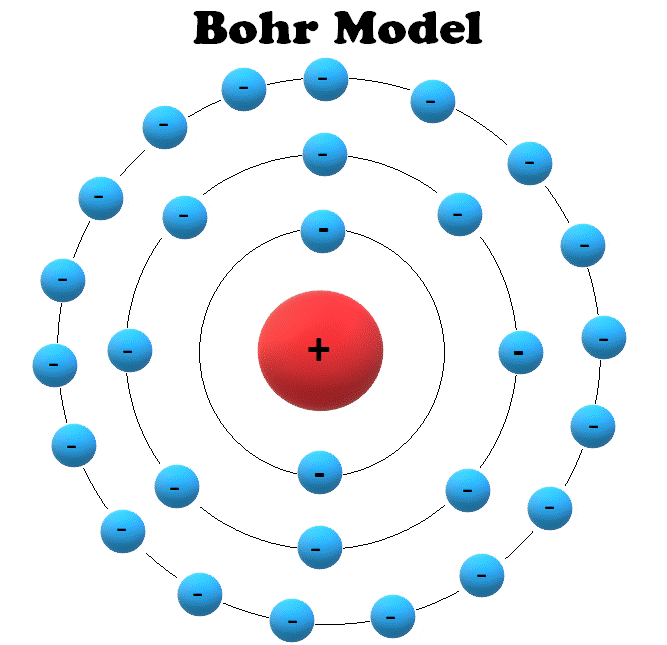
Rutherford explained the nucleus of an atom and Bohr modified that model into electrons and their energy levels. Bohr's model consists of a small nucleus (positively charged) surrounded by negative electrons moving around the nucleus in orbits. Bohr found that an electron located away from the nucleus has more energy, and the electron which.
Bohr's Atomic Model
Resources. Lecture Slides (PDF - 9.3MB) Periodic Table and Table of Constants. Lecture Summary. Prof. Sadoway talks about the principles of modern chemistry and how that led to the understanding of the structure of the atom.He details Bohr's postulates for the hydrogen atom and discusses how the Planck-Einstein relationship applies to electron transitions.

Aluminum Bohr Model Diagram, Using The Main Group Elements Of The Periodic Table To Draw Bohr
A model of a helium atom shows a black circle which fades to white moving from the center to the outside. At the center of the circle is a tiny nucleus, consisting of two red circles and two purple circles.. A Bohr model of a chlorine atom shows a nucleus surrounded by three concentric rings. The ring closest to the nucleus is labelled n=1.

Atom Modelleri(Dalton, thomson, rutherford, bohr atom modelleri) YouTube
1 Ry = e4me 8ϵ2 0h2 = 2.18 × 10 − 18 J. and this simplifies the allowed energies predicted by the Bohr model (Equation 7.4.11) as. En = − (2.18 × 10 − 18)Z2 n2 J = − Z2 n2 Ry. Hence, the energy of the electron in an atom also is quantized. Equation 7.4.12 gives the energies of the electronic states of the hydrogen atom.
Bohr Atom Modeli Fizik. Net. Tr
He was struggling to make sense of all of this. As was common with Bohr when confronted with a puzzle, this struggle was nearly all-consuming. Then in 1913 Bohr, by accident, stumbled across Balmer's numerology for the hydrogen spectrum, and in a flash came up with a workable model of the atom. The model asserts that: The planetary model is.

Atom Modeli ( Bohr Atom Modeli ) YouTube
Bohr Atom Modoli Modern Atom Teorisi (Bulut Modeli) Şimdi bu modelleri sıraysıyla görelim. Bu bir reklamdır: Dalton Atom Modeli (1803) John Dalton John Dalton (1766-1844) yaptığı çalışmalar sonucunda atomu şöyle tarif etmiştir: Maddeler atomlardan oluşur. (Doğru)

Atom Modeli Lityum ( Bohr Atom Modeli ) YouTube
Figure \(\PageIndex{5}\): In Bohr's Model of the atom, electrons absorb energy to move to a higher level and release energy to move to lower levels. (CC BY-SA 3.0; Kurzon). Bohr's Model and Atomic Spectra. The evidence used to support Bohr's model came from the atomic spectra. He suggested that an atomic spectrum is made by the electrons in an.

Oxygen molecule Atom model project, Science projects for kids, Atom model
Bohr's model of the hydrogen atom was the first to incorporate quantum theory, and the key idea of his model was that electrons occupy discrete orbitals. Main Idea A visualization of the Bohr model and the hydrogen spectrum. The Bohr model of the atom was proposed by Niels Bohr in 1913 as an expansion on and correction of the Rutherford model.
mühendislik üçlü Titreme dalton atom modeli şekli
🎈 12. Sınıf Konuları Serisinin Kitabını Sipariş Etmek İçin : https://bit.ly/3xb9oqW🔥 Bu Serinin Oynatma Listesi İçin : https://bit.ly/3B7HFIS🚀 TYT.