Sólidos cristalinos y amorfos explicación, diferencias, ejemplos, etc HiTech

Resources ECE 695A Lecture 5 Amorphous Material/Interfaces Watch Presentation
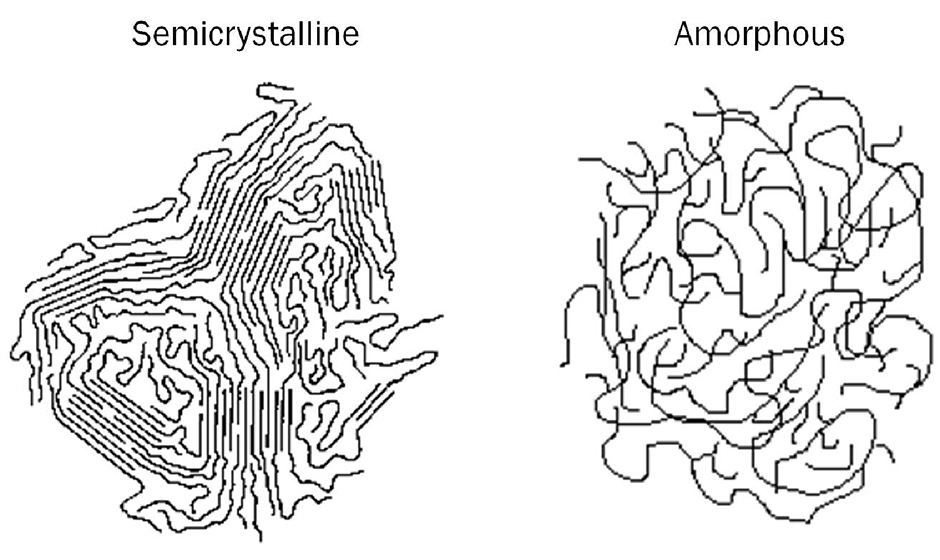
The arrangement of molecular chains in amorphous and semicrystalline polymers. Solidification from the melt Polymers are composed of long molecular chains which form irregular, entangled coils in the melt. Some polymers retain such a disordered structure upon freezing and readily convert into amorphous solids.

PPT Crystallinity in Polymers PowerPoint Presentation, free download ID3117708
Amorphous plastics typically have a shiny and smooth surface finish due to their non-crystalline structure. Some amorphous resins can transmit light which makes them well suited to aesthetic & optical applications. Shrinkage Amorphous plastics generally exhibit low and uniform shrinkage upon cooling.

Sólidos cristalinos y amorfos explicación, diferencias, ejemplos, etc HiTech
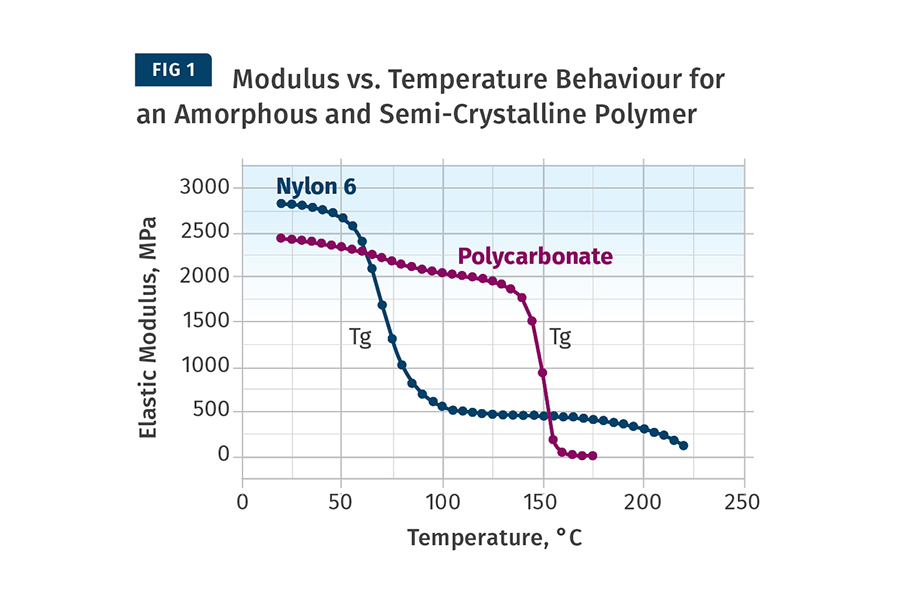
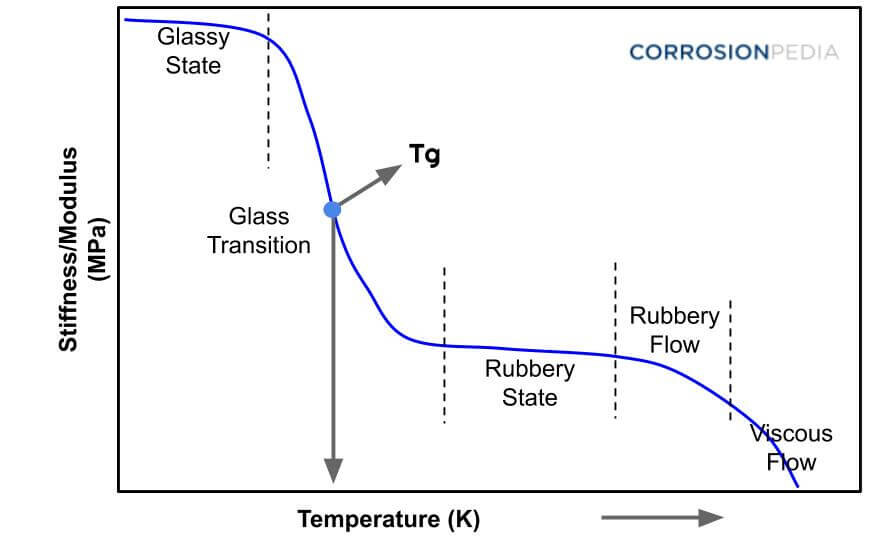
The difference between amorphous & semi-crystalline film. Semi-crystalline films have a highly ordered molecular structure with sharp melt points. While amorphous polymers soften gradually when the temperature rises, semi-crystalline plastics do not. Instead, they remain solid until a certain quantity of heat is absorbed.

Illustration Of A Amorphous Cells Structure Black And White Layered Artwork.
A crystalline polymer is a type of long-chain organic material characterized by the presence of lamellae, which are ordered zones of aligned molecular chains. Crystalline polymers have highly structured regions and can in some cases be entirely composed of a single crystal aligned on one axis. The degree of crystallinity and mutual alignment is.
Basic Approach for the selection of Engineering Plastics
Amorphous PVC to Semi/Crystalline PVC? Hello, Is it possible to convert the structure of PVC Polymer from Amorphous to semi/Crystalline by processing methods in Extrusion?.

Metal Powder Specialist Heraeus Printed 2 kg Amorphous Alloy Gears in 3D SUM provide popular
PVC is highly amorphous, with very low degrees of crystallinity from some syndiotactic polymer sequence as formed in typical commercial process. If the order is forced by polymerization conditions, such as extremely low reaction temperatures (−50 to −100 °C), then a crystalline PVC can be synthesized.
PPT Amorphous Metal Alloys PowerPoint Presentation, free download ID6510039
Amorphous vs. Semi-Crystalline Polymers December 23, 2016 admin No Comments High temperature polymers are divided into two categories: amorphous and semi-crystalline. The difference between the two lies in their molecular structure. In this blog post, we ll discuss how amorphous and semi-crystalline thermoplastics differ from each other.

Xrd Crystallinity Hot Sex Picture
The observed heat of fusion of the mass polymer varied considerably with crystallization temperature, but was in the range 3-5% crystalline, i.e. 0.35-0.55 kJ monomer mol - 1 g: Onset Figure 3 lb Min Isothermal crystallization of PVC Isothcrmat 410 K 2b Metting ~E I I 380 480 K Figure 4 Melting endotherms of PVC 986 POLYMER, 1980, Vol 21.
温度塑料工艺的影响 开云体育登录入口在哪儿下载
The difference between the two lies in their molecular structure. Before you decide which to use, you need to understand the characteristics of each, as that will determine your injection moulding process. In this guide, we'll cover: What is an amorphous polymer? What is crystalline polymer? Examples of amorphous polymers

La différence entre les polymères amorphes et semicristallins Sport and Life
Crystalline and amorphous polymers. Polymers can exist as both crystalline and amorphous solids. In fact, most polymers are semicrystalline, which means that they contain a mixture of crystalline and amorphous regions. In this video, we'll see different examples of semicrystalline and amorphous polymers and learn how their structures can be.

Discussion Which One Of The Following Is Non Crystalline Or Amorphous Updated Sharing Place
As a result, many polymers are semi-crystalline, with regions called lamellae where portions of chains have aligned parallel to each other, but also with large amorphous areas that are much more randomly oriented. As a result, a polymer sample might be 80% amorphous with only 20% of its chain lengths aligned in crystalline lamellae.

15 Schematic cooling (1) and heating (2) DSC curves, showing a range of... Download Scientific
Describe at least two ways to determine experimentally whether a material is crystalline or amorphous. 6. Explain why each characteristic would or would not favor the formation of an amorphous solid. a. slow cooling of pure molten material. b. impurities in the liquid from which the solid is formed.
Why does plastic brittle in cold temperature? Mechanical Engineering Hardware FYI
Polymers with an amorphous morphology have their atoms held together in a loose structure, but this structure is never orderly or predictable, which is why chemists will say that amorphous solids have no long-range order. To understand this better, think of a polymer chain as a piece of spaghetti.
X ray diffraction of semi crystalline and amorphous in Polymer Download Scientific Diagram
It should be noted however, that with both the semi crystalline and amorphous materials at sufficiently high temperature (this is when the material is in its melt state) the molecular structure is amorphous. Table 6.1 classifies some common materials into these two groups.

Crystalline vs Amorphous Difference and Comparison
AMORPHOUS POLYMERS - are characterized by having a disorganized pattern of polymers. The polymer chains are disoriented, random in length, and intertwined like a bowl of pasta. Amorphous comes from the Greek word for "shapeless." The random structure has definite advantages in terms of properties.

Courses nanoHUBU Primer on Semiconductor Fundamentals Fall 2018 (SelfPaced)
The lattice of crystalline quartz (SiO 2).The atoms form a regular arrangement in a structure that consists of linked tetrahedra. In an amorphous solid, the local environment, including both the distances to neighboring units and the numbers of neighbors, varies throughout the material.