PPT The History of the Atom Timeline PowerPoint Presentation ID2061928
Evolution of idea of an atom Atomic Structure Term 1 Unit 4 7th Science
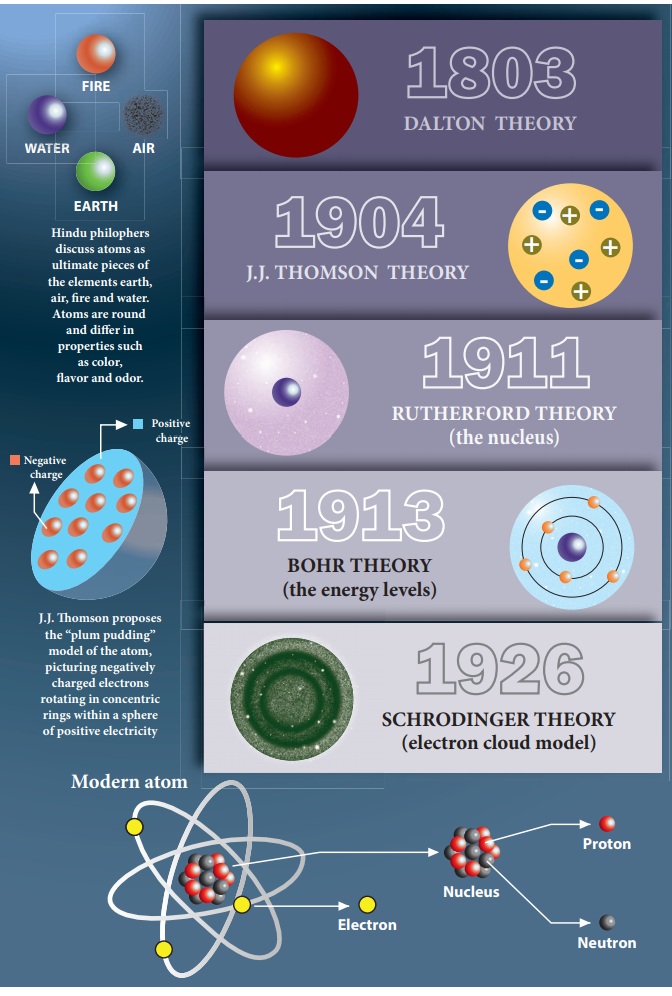
The structure of atoms is somehow related to electricity. (p.95) Discovered atoms have negative particles (electrons) using a cathode ray tube. Discovered electron's charge to mass ratio: 1.76 x 108 C/g. (p. 97-98) Thomson's Plum Pudding Model, 1900.
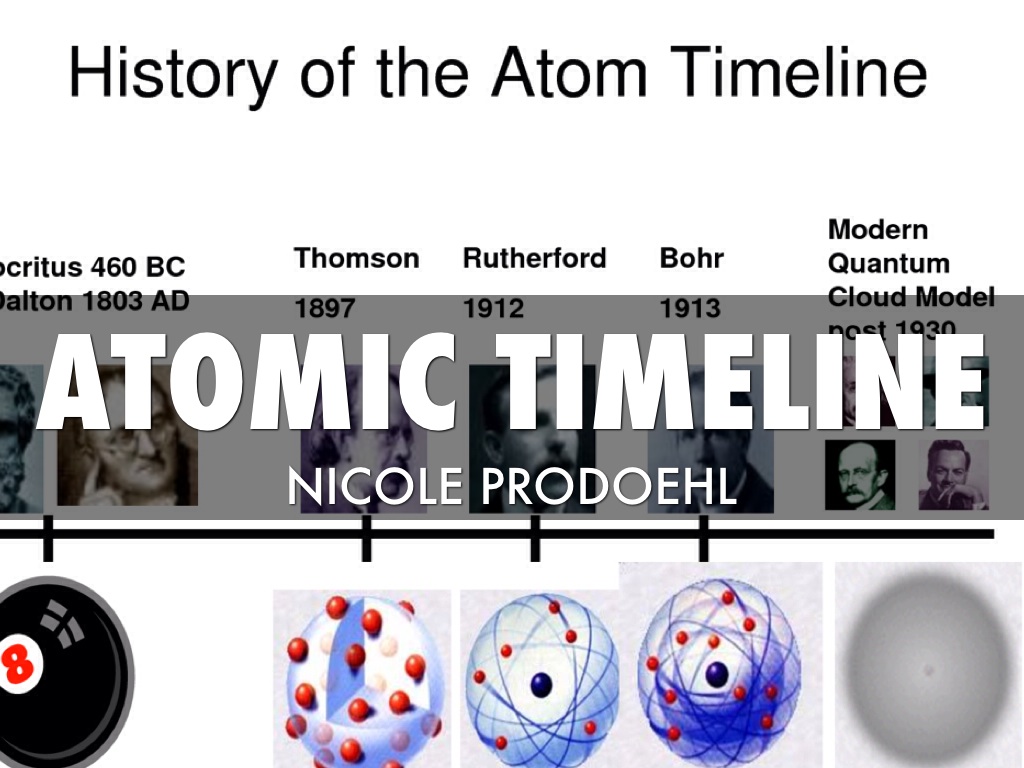
Atomic Timeline Nicole Prodoehl by Nicole Prodoehl
Matter is composed of exceedingly small particles called atoms. An atom is the smallest unit of an element that can participate in a chemical change. An element consists of only one type of atom, which has a mass that is characteristic of the element and is the same for all atoms of that element (Figure 2.1.1. 2.1. 1.
Hazel Azaña
1803 John Dalton introduces atomic ideas into chemistry and states that matter is composed of atoms of different weights 1805 (approximate time) Thomas Young conducts the double-slit experiment with light 1811 Amedeo Avogadro claims that equal volumes of gases should contain equal numbers of molecules
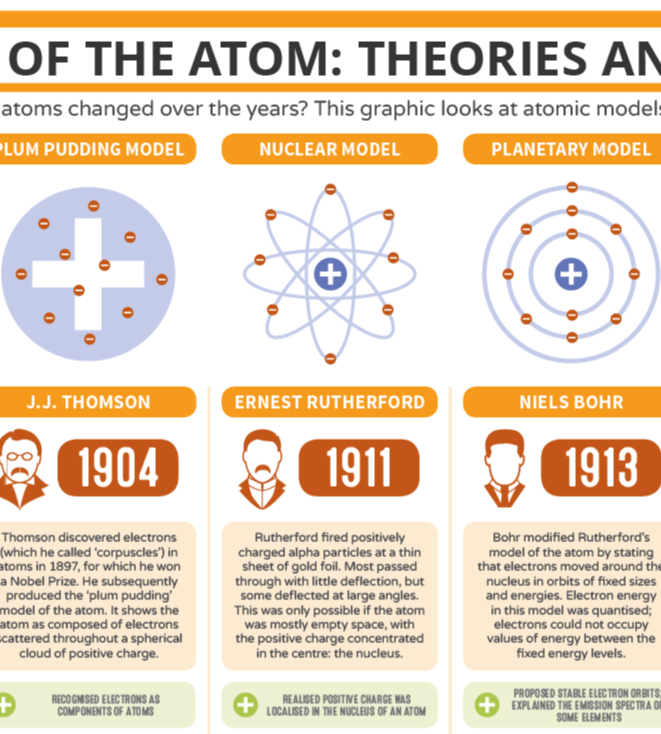
A History of the Atom Theories and Models
1776 - 1844 He is from Cumberland, England. His atomic theory said that elements consisted of tiny particles called atoms. It states an element is one of a kind (aka pure) because all atoms of an element are identical. All the atoms that make up the element have the same mass. All elements are different from each other due to differing masses.

The History of the Atom Theories and Models Compound Interest
To see all my Chemistry videos, check outhttp://socratic.org/chemistryThis video is about the different ways that scientists have pictured the atoms over the.
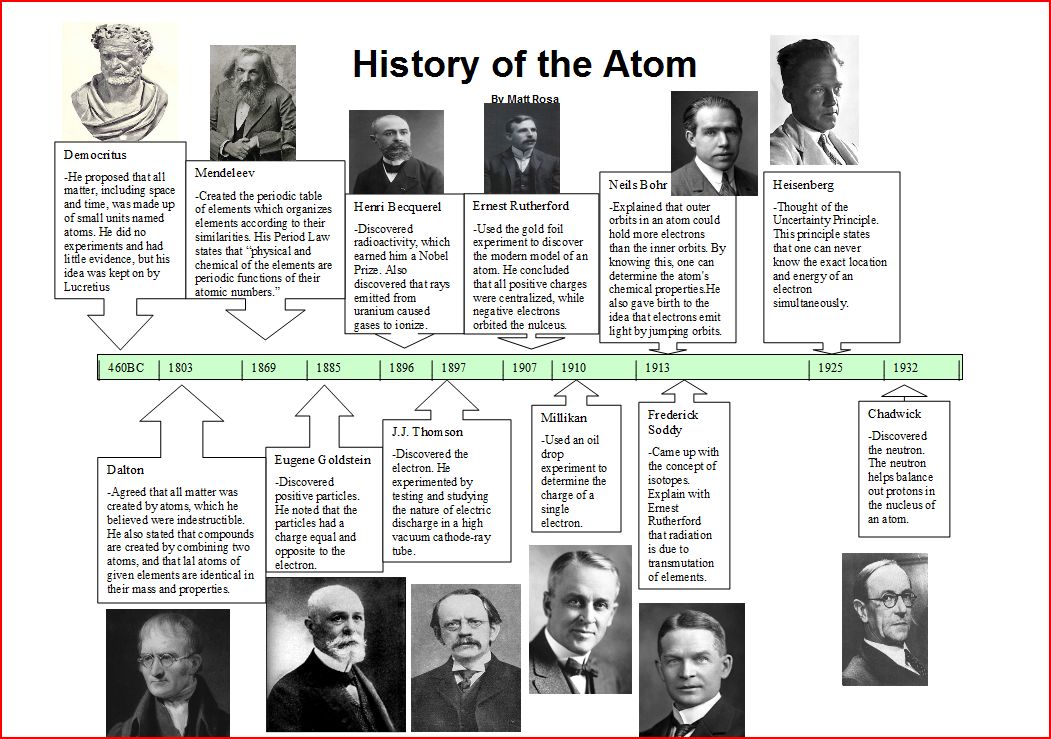
Slide 3
Atomic model, in physics, a model used to describe the structure and makeup of an atom. Atomic models have gone through many changes over time, evolving as necessary to fit experimental data. For a more in-depth discussion of the history of atomic models, see atom: development of atomic theory.

Atomic theory on Pinterest Atoms, History and Chemistry
History of the Atom Timeline. Scientist name Timeframe Major Discovery; Democritus: 400 B.C. The smallest indivisible particle of matter is called an atom. Dalton: 1803: All elements are made of.

PPT The History of the Atom Timeline PowerPoint Presentation ID2061928
Physics The Discovery of the Atom What would happen if we took a piece of paper and repeatedly cut it in half? Eventually, it would become impossible to continue with regular tools such as scissors, as the piece would simply be too small.

Timeline Of Atomic Models Stock Illustration Download Image Now Atom, Model Object
All matter is made up of atoms. This is something you learn right back at early chemistry classes. Despite this. our new ideas about what an atom is. is surp.

Atomic timeline Atom, Atomic theory, Timeline
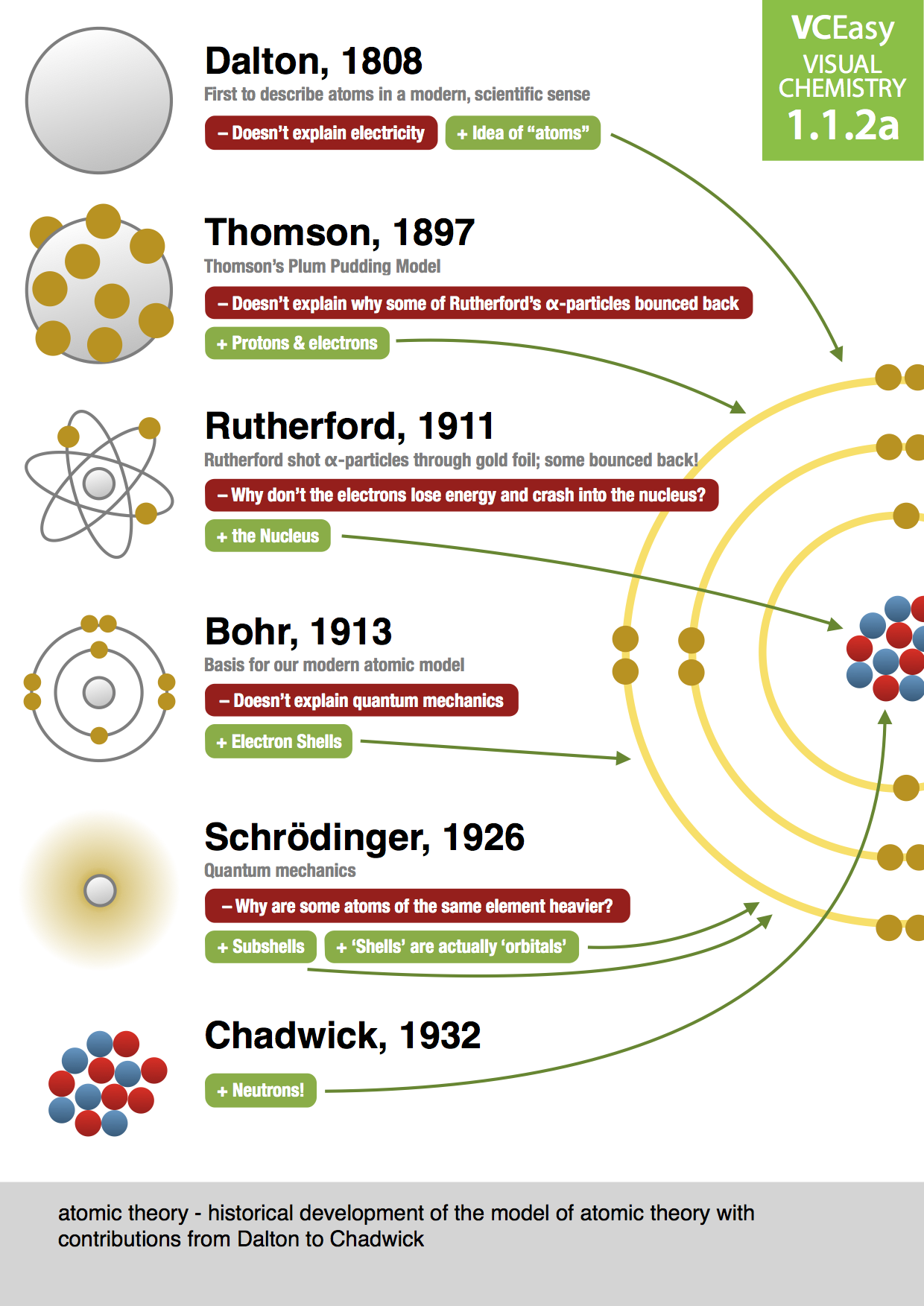
In 1803, John Dalton presented his atomic theory based on three key ideas: Matter is made of atoms which are tiny particles that cannot be created, destroyed, or divided Atoms of the same element are identical, and atoms of different elements are different Different atoms combine together to form new substances
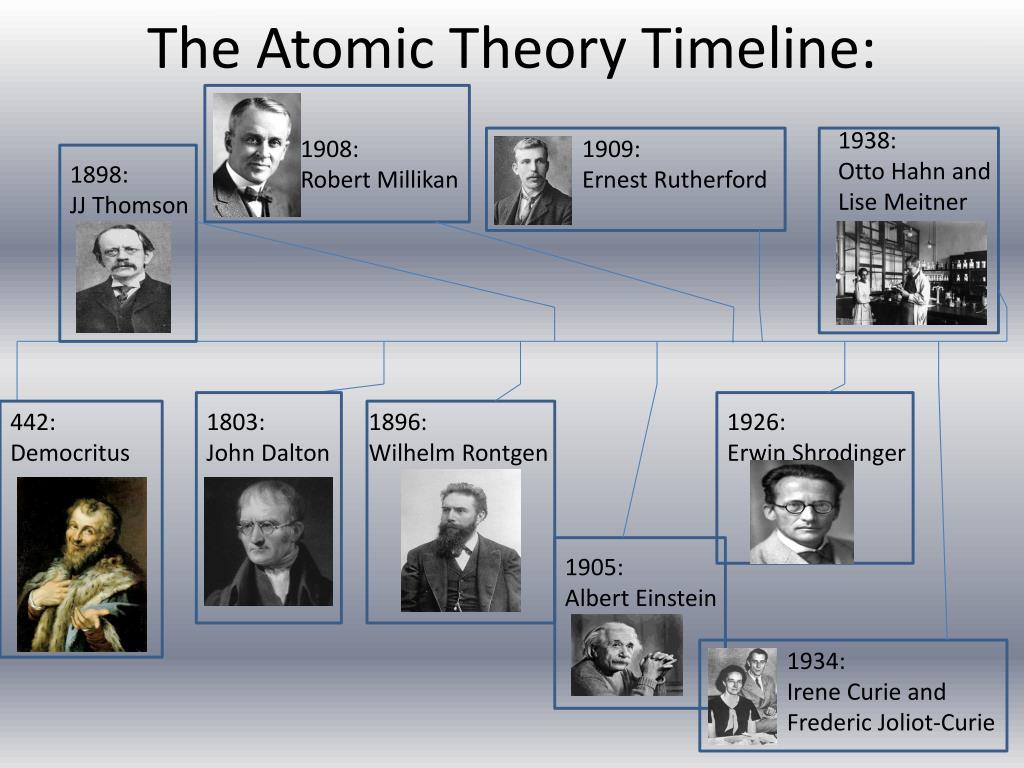
PPT The Atomic Theory Timeline PowerPoint Presentation, free download ID1854005
. Later Greek thinkers suggested that matter could be made up of invisible particles . They called these particles atoms but they had no experimental evidence for their model. The first atomic.

History Of Atoms Timeline boostermash
In this video you will learn all the science for this topic to get a grade 9 or A* in your science exams! History Of The Atom Timeline - GCSE Chemistry | kay.

history of the atom with timeline Atoms Electron
It wasn't until 1803 that the English chemist John Dalton started to develop a more scientific definition of the atom. He drew on the ideas of the Ancient Greeks in describing atoms as small, hard spheres that are indivisible, and that atoms of a given element are identical to each other.
PPT History of the Atom Timeline PowerPoint Presentation, free download ID1169012
Matter is composed of exceedingly small particles called atoms. An atom is the smallest unit of an element that can participate in a chemical change. An element consists of only one type of atom, which has a mass that is characteristic of the element and is the same for all atoms of that element (Figure \(\PageIndex{1}\)). A macroscopic sample.
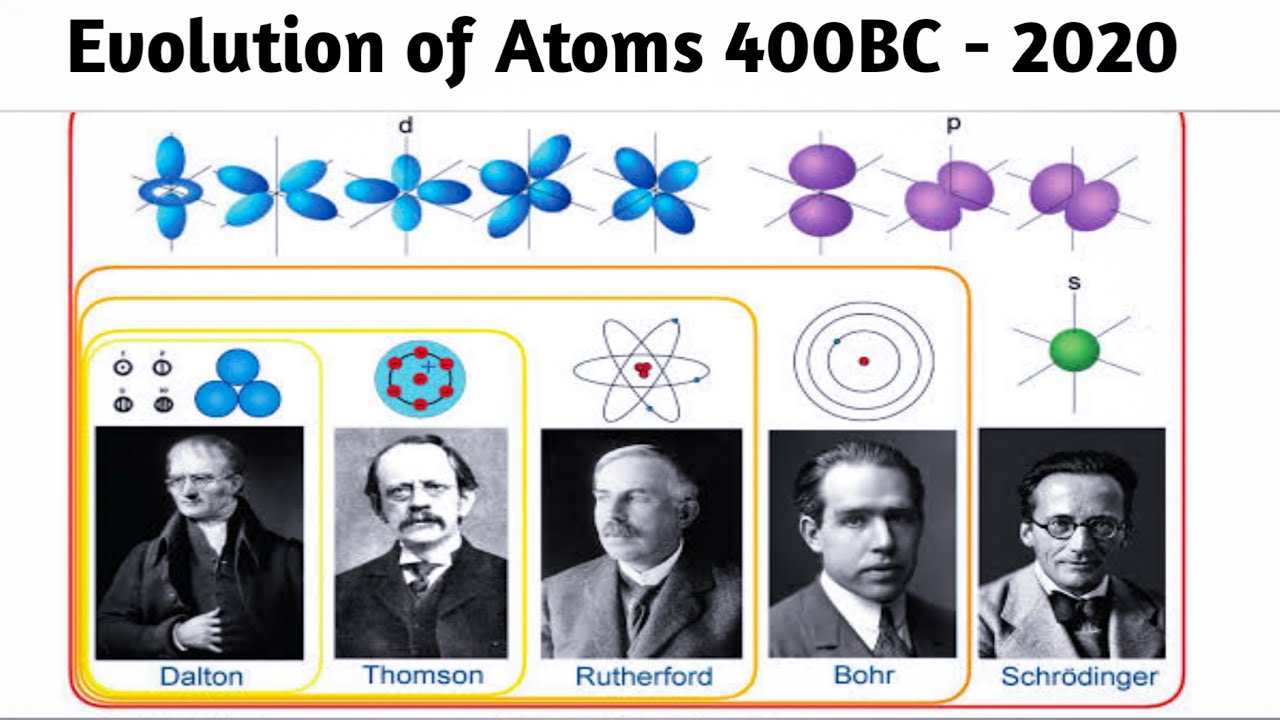
Evolution of Atomic Model 400 BC 2020 History of the atom Timeline, Atomic Theories YouTube
Center for History of Physics Teaching Guides The Evolution of Atomic Theory The Evolution of Atomic Theory J.J. Thomson and Ernest Rutherford conversing, Cavendish Laboratory, University of Cambridge. Photograph by D. Schoenberg, courtesy of AIP Emilio Segrè Visual Archives, Bainbridge Collection.

Discovery of the Atom Timeline YouTube
Leucippus of Miletus (5th century bce) is thought to have originated the atomic philosophy. His famous disciple, Democritus of Abdera, named the building blocks of matter atomos, meaning literally "indivisible," about 430 bce.