Solved Give the systematic name for the following

Provide The Correct Iupac/Systematic Name For The Following Compound.
This nomenclature will be discussed when it is possible for a functional group. 3.2: Overview of the IUPAC Naming Strategy is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The International Union of Pure and Applied Chemistry (IUPAC) names for organic compounds all follow the same set of rules.
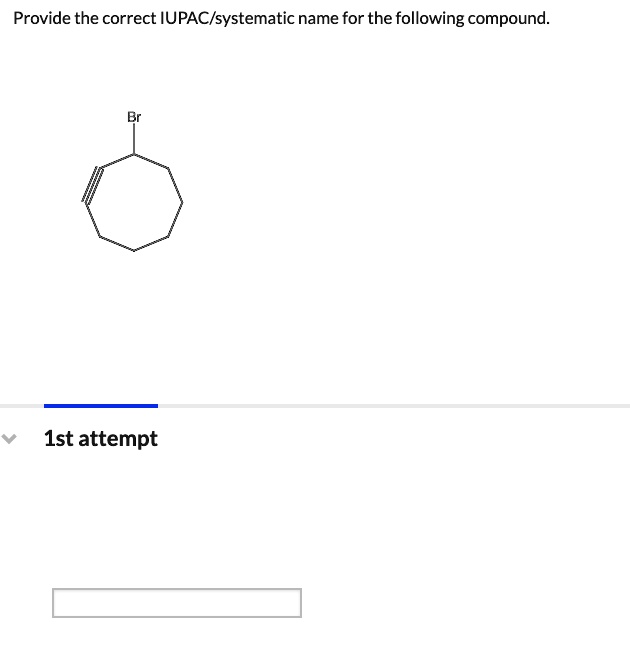
SOLVED Provide the correct IUPAC/systematic name for the following compound. 1st attempt
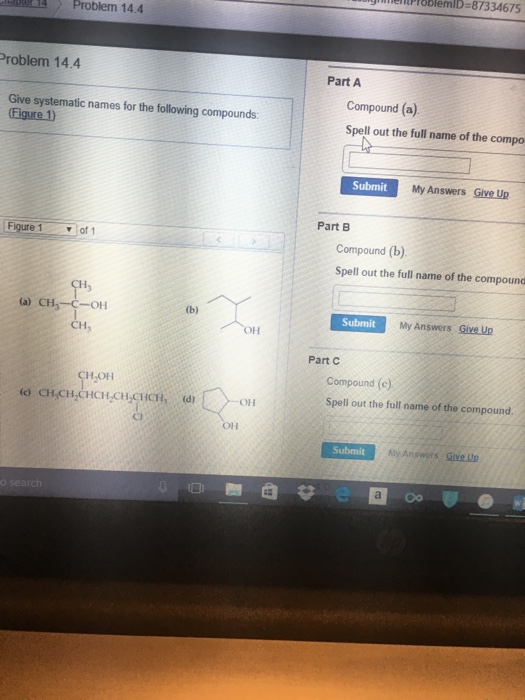
Question: Give systematic names for the following compounds. (Figure 1) Compound (a). Spell out the full name of the compound. Compound (b). Spell out the full name of the compound. Compound (c). Spell out the full name of the compound. Show transcribed image text Here's the best way to solve it. Expert-verified 100% (1 rating) Share Share
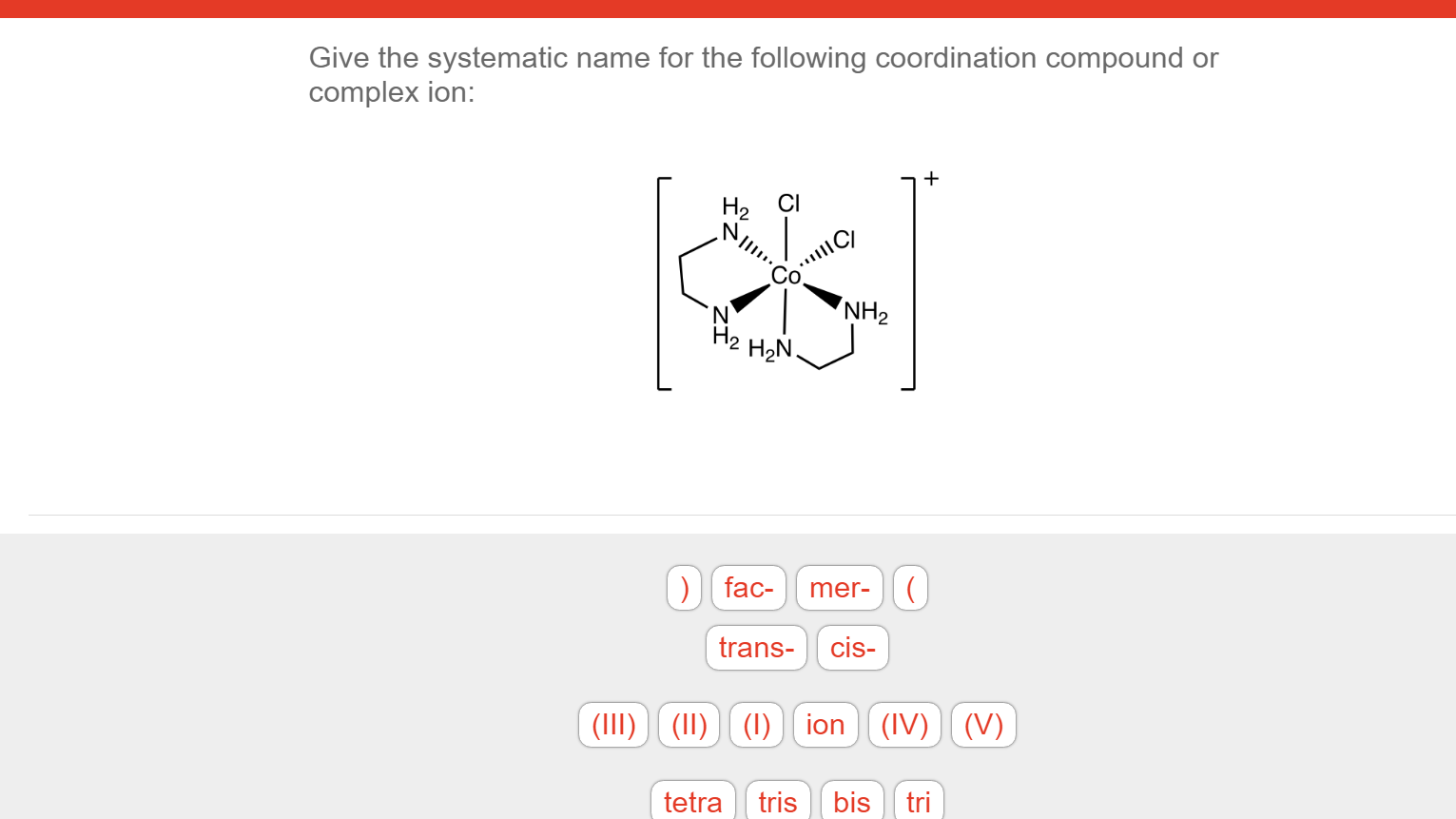
Solved Give the systematic name for the following
For many compounds, the systematic name and the common name are both used frequently, requiring familiarity with both. For example, the systematic name for NO is nitrogen monoxide, but it is much more commonly called nitric oxide. Similarly, N 2 O is usually called nitrous oxide rather than dinitrogen monoxide.
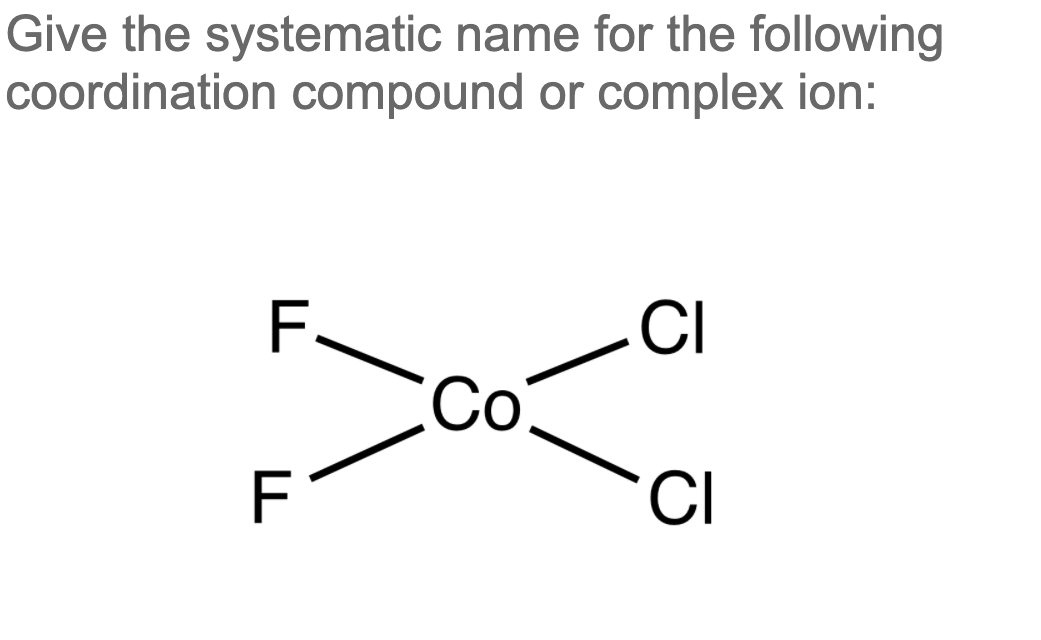
Solved Give the systematic name for the following
All of the following names represent a compound that has been named improperly. Draw out the structure from the name and give the proper IUPAC name for the compounds. 1,3-dimethylbutane; 4-ethylpentane; 2-ethyl-3-methylpentane; Answer. a) The structure that can be drawn for the improper name is shown below on the left.
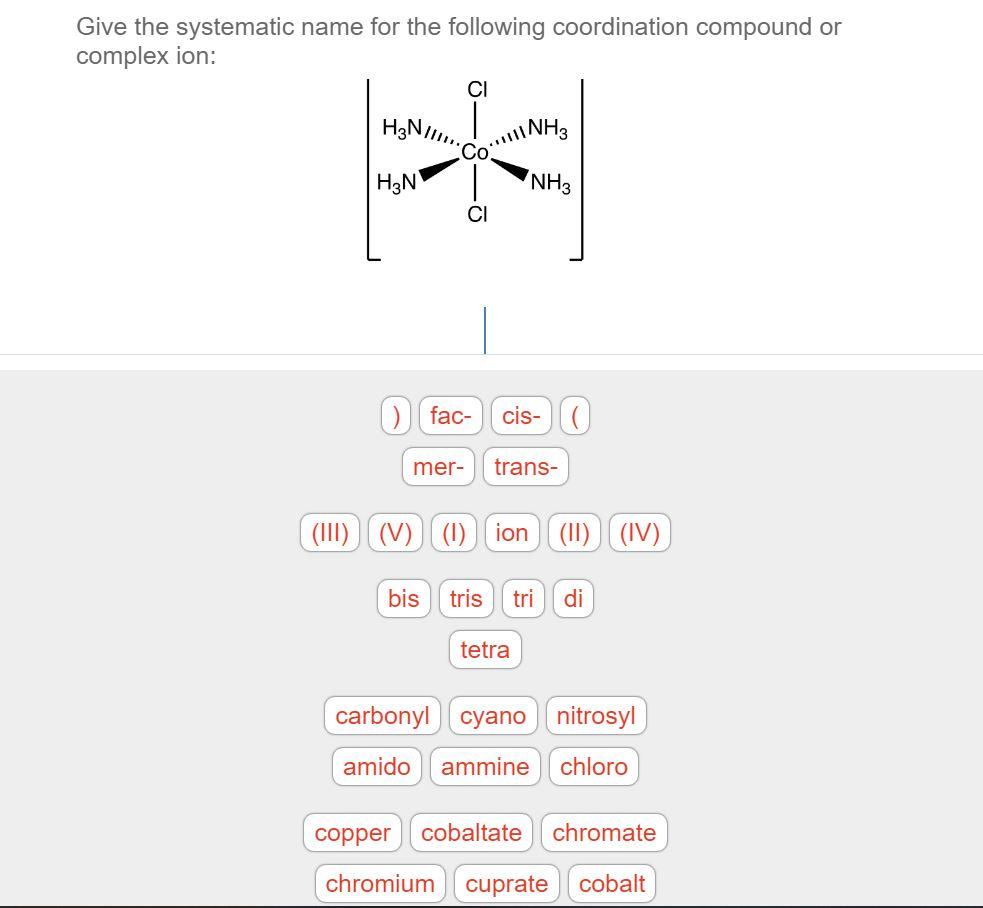
Solved Give the systematic name for the following
This is a set of practice problems on naming organic compounds. The examples cover the nomenclature of alkanes, bicyclic compounds, alkenes, alkynes, alcohols, alkyl halides, aromatic compounds, aldehydes and ketones, amines, ethers, and carboxylic acid derivatives such as nitriles, esters and amides.

OneClass Part F Give systematic (IUPAC) name for the following compound Br Spell out the full
Table 6.1 lists the common (trivial) names of some molecular compounds. Several ionic compounds are listed in Table 6.2, with both their common and systematic names. TABLE 6.2 Names and formulas of some common ionic compounds. Common name. Systematic name. Formula. bleach. sodium hypochlorite. NaOCl.
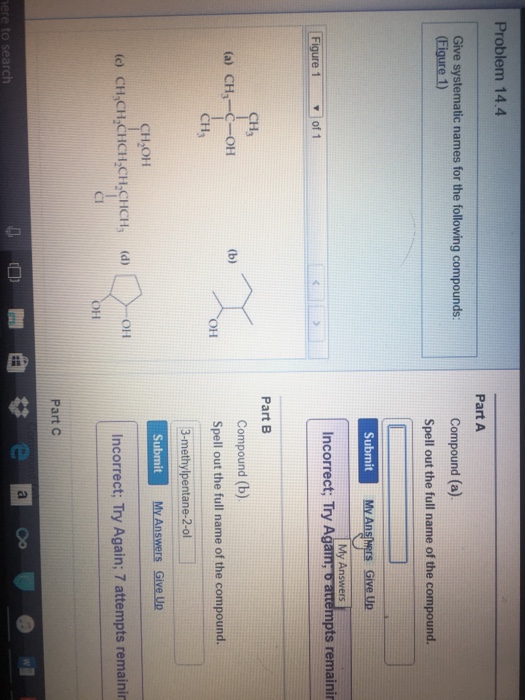
Solved Give systematic names for the following compounds.
Give the systematic name for each of the following compounds: 1. 2. CH3CH2C (CH3)3 CH3 CH3 CH_CH_CHCH_CCH CH; 3. . CH:CH-C (CH2CH3)2CH2CH2CH3 4. CH3 CH2CHCH,CH,CHCH CH2CH3 . 5. CH3CH2C (CH2CH3)2CH (CH3)CH (CH2CH2CH3)2 6.
Solved Give systematic names for the following compounds
Example 10.3.1. Write the systematic name (and the common name if applicable) for each ionic compound. LiCl; MgSO 4 (NH 4) 3 PO 4; Cu 2 O; Given: empirical formula Asked for: name Strategy: A If only one charge is possible for the cation, give its name, consulting Table 10.1.2 or Table 10.2.1 if necessary. If the cation can have more than one charge (Table 10.3.1), specify the charge using.
Solved Give the systematic name for the following
Notice that the mono-prefix is not used with the nitrogen in the first compound, but is used with the oxygen in both of the first two examples. The \(\ce{S_2Cl_2}\) emphasizes that the formulas for molecular compounds are not reduced to their lowest ratios. The o of the mono-and the a of hepta-are dropped from the name when paired with oxide.
Solved Give the systematic name for the following
Here are the rules for naming binary covalent compounds. A binary compound is one that consists of only two elements. The names are called systematic names. First, name the nonmetal furthest to the left and bottom of the periodic table by its element name. Second, name the other nonmetal by its element name, but shorten its name and add an -ide.

Solved Give the systematic name for the compound
The longest chain containing the double bond has five carbon atoms, so the compound is a pentene (rule 1). To give the first carbon atom of the double bond the lowest number (rule 2), we number from the left, so the compound is a 2-pentene. There is a methyl group on the fourth carbon atom (rule 3), so the compound's name is 4-methyl-2-pentene.

Solved Give systematic names for the following compounds
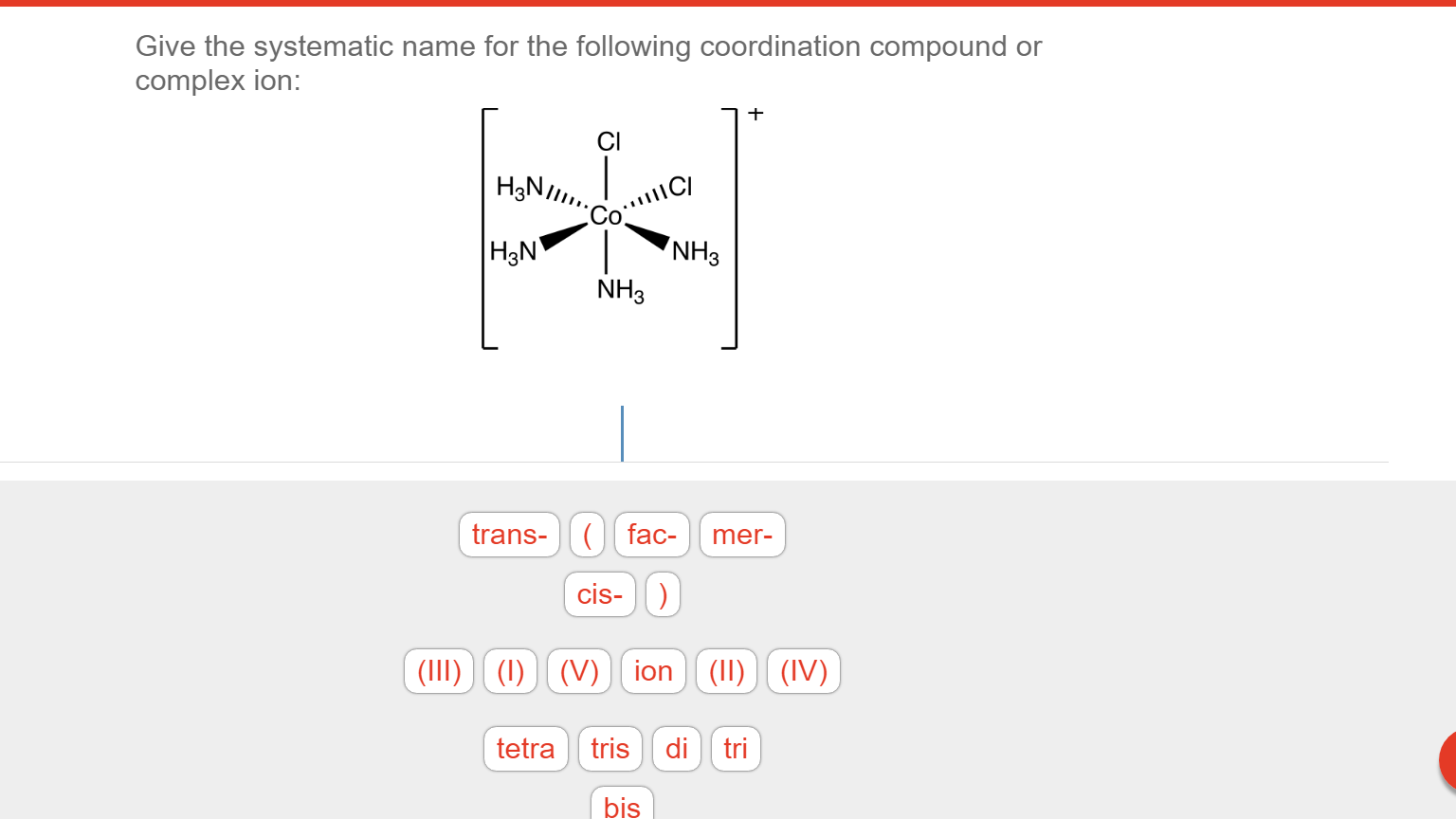
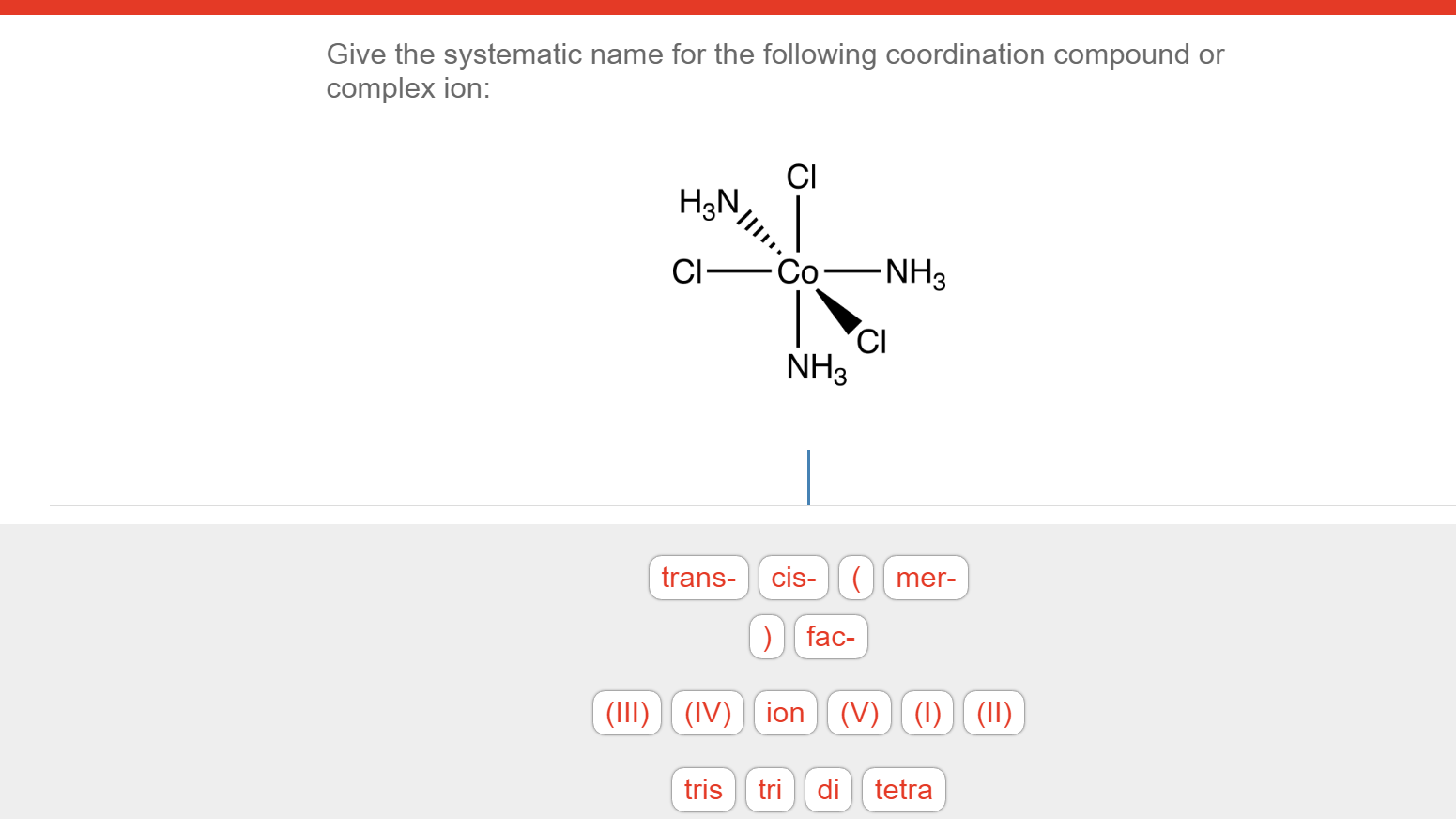
There are 2 steps to solve this one. Expert-verified 100% (2 ratings) Step 1 To name a coordination complex, follow these steps: Identify the cation and write its name first. Thi. View the full answer Step 2 Unlock Answer Unlock Previous question Next question Transcribed image text:
[Solved] Provide a systematic name for the following compound.... Course Hero
What is the systematic name of the following compound? Al 4 C 3 Choose 1 answer: Silver carbonate A Silver carbonate Silver carbide B Silver carbide Aluminum carbide C Aluminum carbide Aluminum carbonate D Aluminum carbonate Show Periodic Table Stuck? Use a hint. Report a problem Do 4 problems
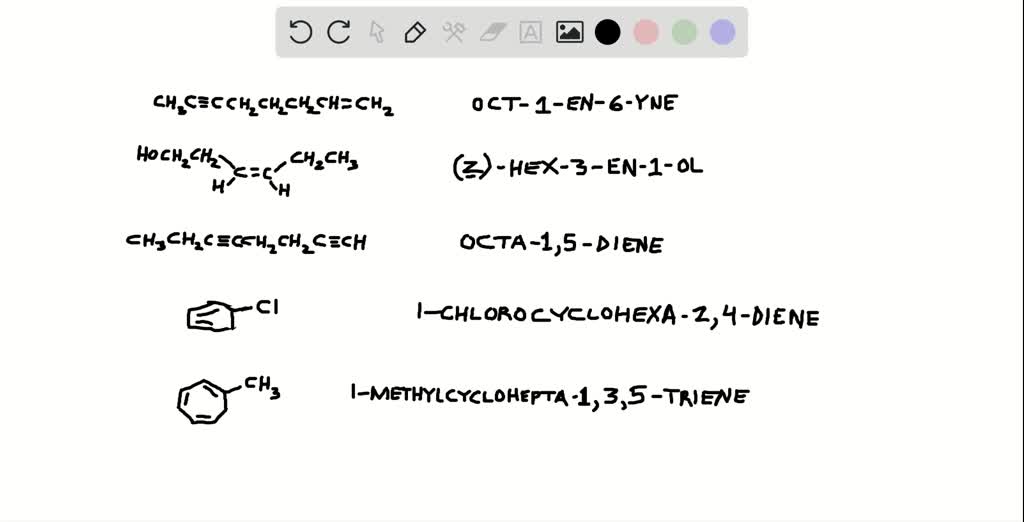
SOLVEDGive the systematic name for each of the following compounds
Learn Test Match Q-Chat Created by carrots92 Terms in this set (6) Classify each of the following compounds as ionic or molecular. HCN CCl4 NI3 PtO2 Cr2O3 Ionic: PtO2 Cr2O3 Molecular: HCN CCl4 NI3 Give the systematic name for the compound Mg (NO3)2.
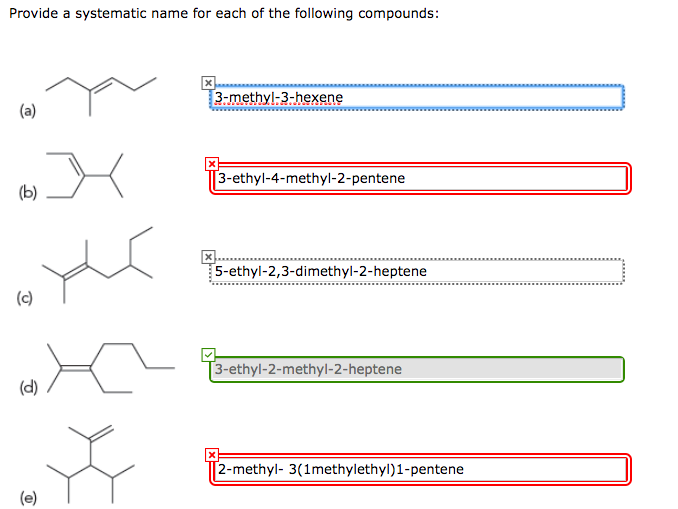
Provide a Systematic Name of the Following Compound Below MarenhasColeman
In this task, we need to give a systematic name to three different compounds. All three elements (nitrogen, oxygen and sulfur) are nonmetals, so we need to list the rules for naming molecular covalent compounds: The first element in the formula is named using the full element name. The second element is named as an anion with the suffix -ide.
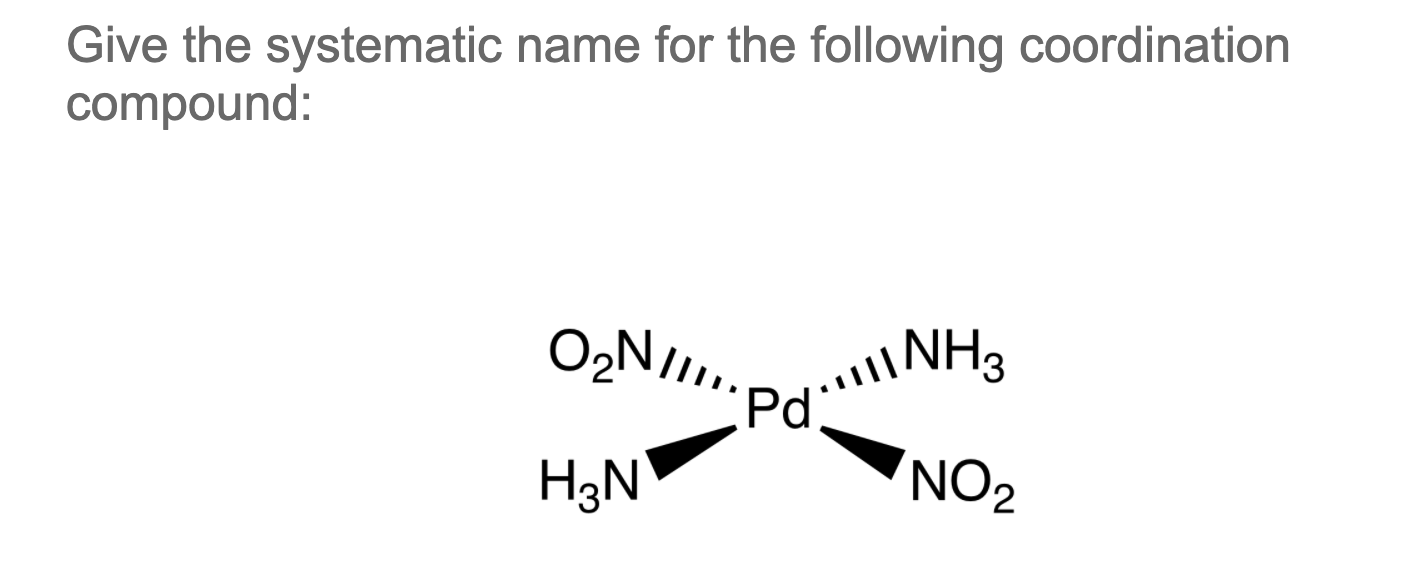
Solved Give the systematic name for the following
She selects test tubes from the first 8 8 experiments performed on Tuesday. chemistry. Give the systematic name for each of the following compounds: (a) Cu2S and CuS, (b) Na2SO4, (c) As4O6, (d) ZrC14, (e) Cl2O7, (f) Ga2O. 1 / 4. Find step-by-step Chemistry solutions and your answer to the following textbook question: Give systematic names for.